
The top three Prescribed Perspectives articles downloaded by health systems in June.
Six important actions to take ahead of the upcoming DSCSA implementation
By
Date
November 07, 2023

Read time: 3 minutes
By: McKesson Health Systems Editorial Team, Scott Mooney
The industry has been preparing for the Drug Supply Chain Security Act (DSCSA) implementation for the past 10 years, with the legal deadline approaching on November 27, 2023. On Friday, August 25, 2023, the U.S. Food and Drug Administration (FDA) announced through a compliance policy that it would not take action to enforce the Enhanced Drug Distribution Security requirements of the Drug Supply Chain Security Act (DSCSA), section 582(g)(1) of the Federal Food, Drug, and Cosmetic Act, until November 27, 2024.
The FDA is providing a one-year stabilization period (“Stabilization Period”) beyond the legal deadline of November 27, 2023. This period will allow all key players within the supply chain (manufacturers, distributors, dispensers, and trading partners) to mature processes and refine operations that are required to comply with these specific package-level tracing requirements. The FDA will continue to enforce the other DSCSA requirements during this Stabilization Period.
As your trusted wholesaler, McKesson will provide guidance to help prepare you for DSCSA implementation. There are actions that you must take to ensure that you are fully compliant and prepared for DSCSA implementation. The graphic below provides vital details you need to know.
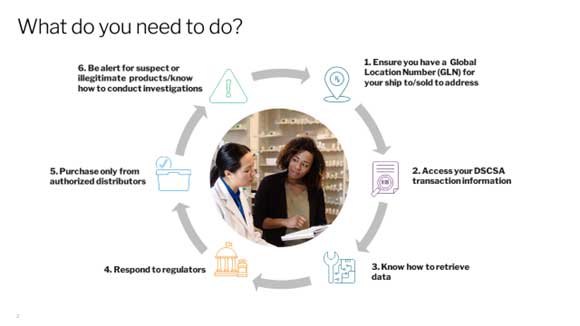
1. You must have access to your historical DSCSA transaction data. In the near future, your DSCSA transaction data will be accessible through the backend of McKesson ordering portals or in your in-house or third-party systems if you request information to be transferred there.
2. You should be prepared to track ownership of a product and respond to regulators. All trading partners, including dispensers, should be ready to respond directly to regulators if asked about serial DSCSA transaction information and statements upon request. McKesson is not authorized to speak on your behalf but will have the information readily available via the ordering portals or within your in-house or third-party systems.
3. Be alert for Suspect Products and have procedures in place to identify them according to DSCSA requirements. As a dispenser, you should evaluate all product within your possession and control for suspect product indications, including but not limited to incorporating monitoring of the bar code and serial number into your processes and sharing information with up and downstream trading partners. Have a standard operating procedure for your suspect product.
4. You are in charge of reporting to the FDA. If, after conducting an investigation, you find that a product is illegitimate, you will need to report the issue to the FDA and notify your trading partners.
5. Please watch the latest episode of our new DSCSA webisode series featuring our McKesson and DSCSA industry expert, Scott Mooney, who will thoroughly explain the actions you need to take to prepare for DSCSA implementation.

6. Continue to prepare for November 27, 2023. As your trusted partner, we encourage you to continue refining your processes to prepare for DSCSA implementation. The FDA expects all parties to continue progressing toward stabilization and maturing systems during the one-year Stabilization Period. The FDA will continue to enforce the other DSCSA requirements during this Stabilization Period. We will be with you every step of the way as you prepare for implementation.
We will share more information and videos in the coming months to assist you. If you have additional DSCSA-related questions, please contact your McKesson sales contact or Customer Support today.